What Are 3 Common Properties Of Covalent Compounds
Color and odor are physical properties. Lets look at some of the more common physical properties of compounds.
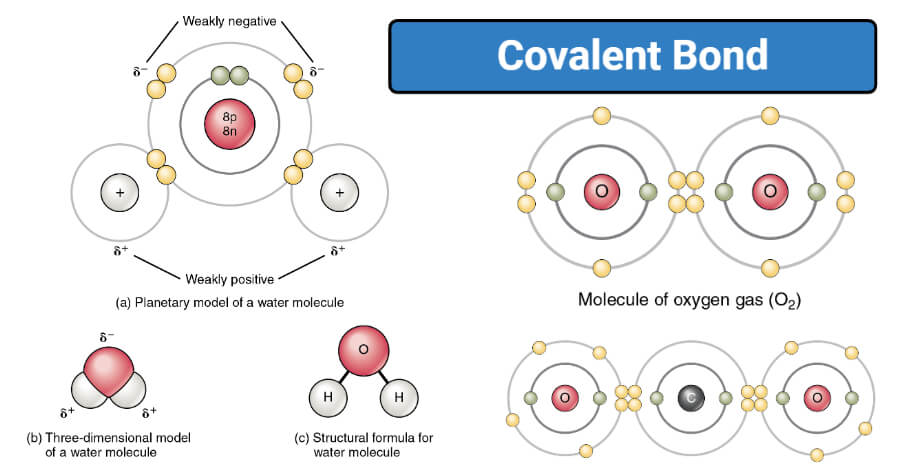
Covalent Bond Definition Properties Types Formation Examples
The compound carbon disulfide CS 2 is described with the structural formula ie.

What are 3 common properties of covalent compounds. Properties of Ionic and Covalent Compounds. Common table salt is one example of this kind of solid. 38 Properties and Types of Ionic Compounds Ionic compounds are held together by the electrostatic forces created by the attraction of the positively charged cations and negatively charged anions.
When dissolved in water they dont conduct electricity. In crystalline solids the atoms ions or molecules are arranged in an ordered and symmetrical pattern that is repeated over the entire crystal. These can be simple ions such as the sodium Na and chloride Cl in sodium chloride or polyatomic species such as the ammonium NH 4.
Covalent compounds tend to be more flammable than ionic compounds. Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds. This molecule is not a disulfide in the sense that.
Cations are ions which have a positive electrical chargeA cation has fewer electrons than protons. The properties of ionic compounds relate to how strongly the positive and negative ions attract each other in an ionic bond. Disulfide is also used to refer to compounds that contain two sulfide S 2 centers.
Iconic compounds also exhibit the following properties. The mixture of nitric and hydrochloric acids was known as aqua regia royal water celebrated for its ability to dissolve. There are many types of physical properties that can be used to tell compounds apart.
An ion may consist of a single atom of an element a monatomic ion or monatomic cation or anion or of several atoms that are bonded together a polyatomic ion or polyatomic cation or anionBecause of their net electrical charge cations are repelled by other cations and are attracted to. Compounds with three sulfur atoms such as CH 3 SSSCH 3 are called trisulfides or trisulfide bonds. A Common Household Example.
Nitrogen compounds have a very long history ammonium chloride having been known to HerodotusThey were well known by the Middle Ages. Maybe youll be interested in comparison of properties of ionic and covalent compound so here is a link where you can learn it. Alchemists knew nitric acid as aqua fortis strong water as well as other nitrogen compounds such as ammonium salts and nitrate salts.

Covalent Bond Definition Types And Examples

Covalent Bond Definition Types And Examples

Ionic Bonds Vs Covalent Bonds Chemtalk
What Is A Covalent Bond A Covalent Bond

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
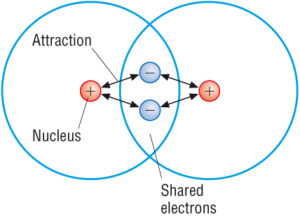
Covalent Compounds Covalent Bond Properties Examples With Videos

Covalent Bond An Overview Sciencedirect Topics

7 Some Of The Properties Associated With Many Simple Ionic And Covalent Download Scientific Diagram

Single Covalent Bond Definition And Examples

Covalent Bond Examples Formation Properties What Is A Covalent Bond Video Lesson Transcript Study Com

Covalent Bond Definition Types And Examples
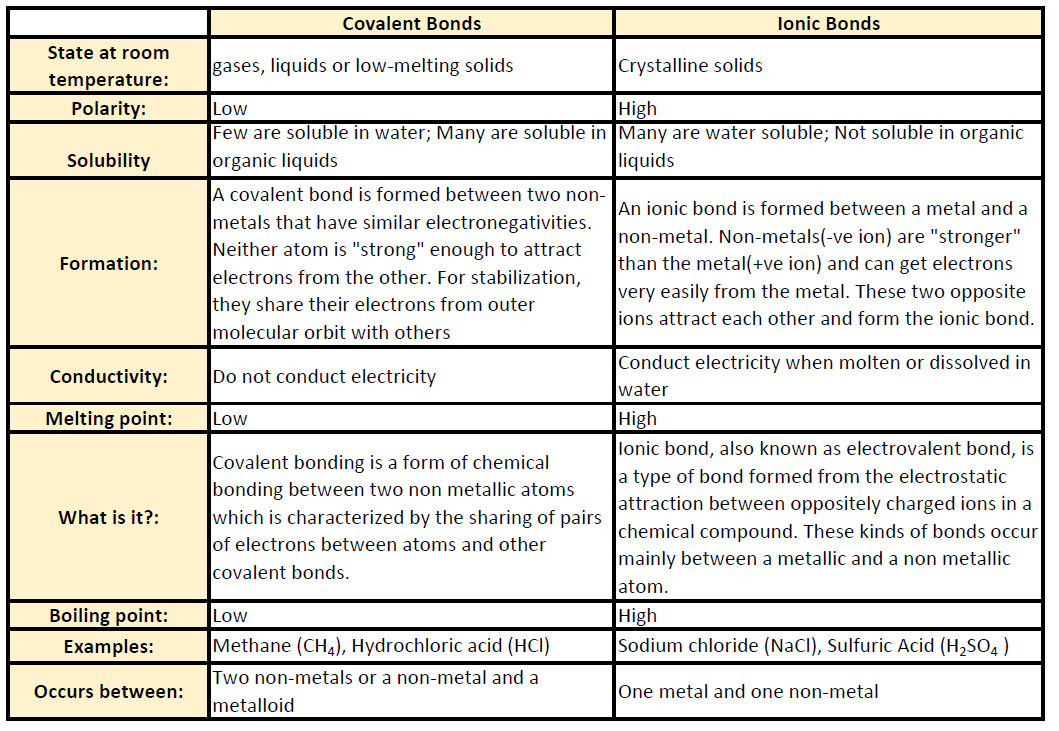
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bond Definition Properties Examples Facts Britannica

Science Comicz Ionic Covalent Bonding Storyboard
What Does A Covalent Bond Mean Quora

6 2 Covalent Compounds Pages Learning Goals I Can Explain How Covalent Compounds Are Formed I Can Describe The Properties Of Covalent Compounds Ppt Download
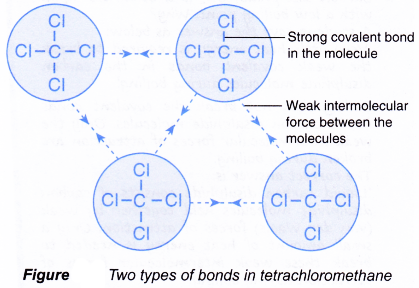
Properties Of Ionic And Covalent Compounds A Plus Topper

Covalent Compounds Covalent Bond Properties Examples With Videos

Covalent Bonds Biology For Majors I