5 Examples Of Inorganic Compounds
Exceptions Few carbon containing chemical compounds like metal cyanides CN oxides of carbon CO2 CO. Homogenous mixtures and heterogeneous mixtures.

Inorganic Compounds Anatomy And Physiology
Discover the characteristics applications and examples of these compounds.

5 examples of inorganic compounds. And some examples of ketones are propanone acetone 2-methyl-3-pentanone etc. The examples of aldehydes include propanol butanol 4-chlorobutanol etc. Learn more about the definition of.
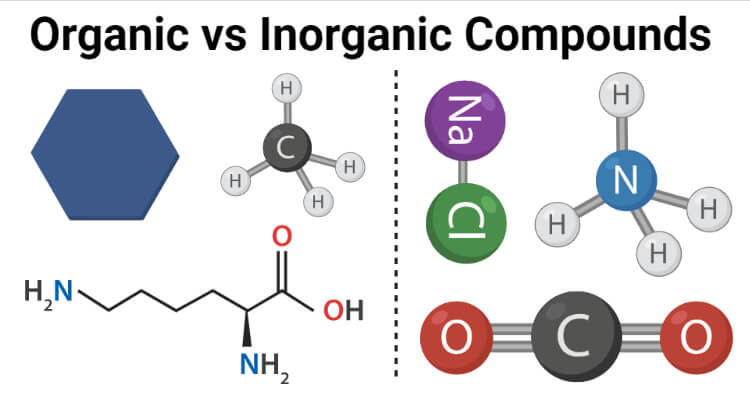
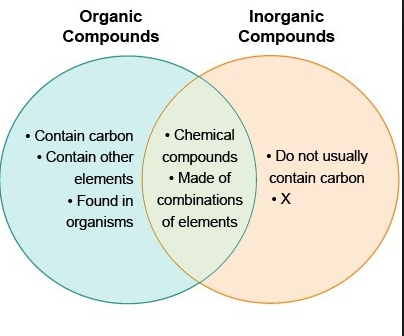
Such as methane CH4 ethane C2H6 benzene C6H6 etc. H 2 O - Water is a simple inorganic compound even though it contains hydrogen a key atom along with carbon in many organic compounds. Compounds can be classified as organic compounds or inorganic compounds depending on the presence of carbon in the molecular structure.
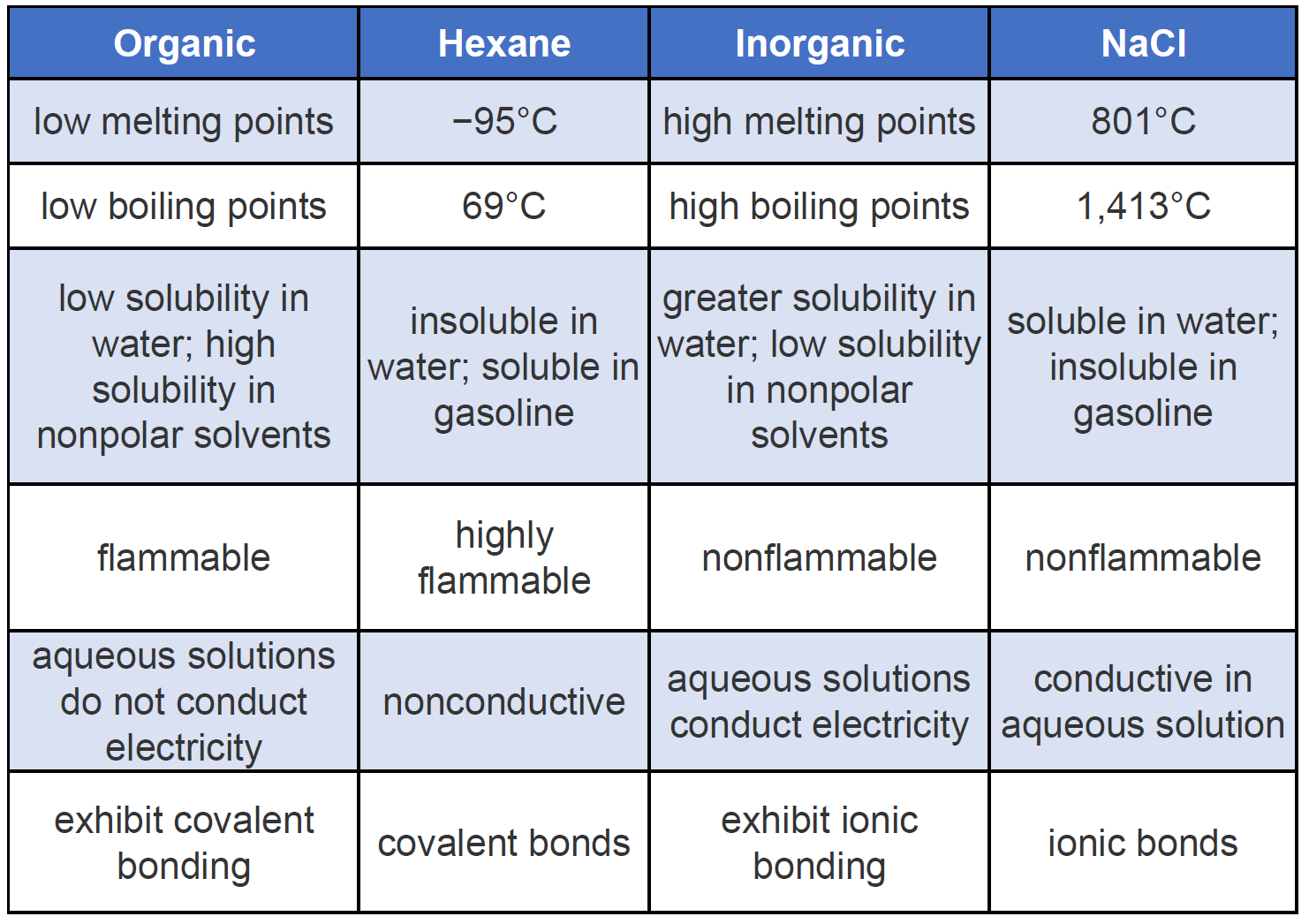
Some properties of carbonyl compounds are given below. You can recognize ionic compounds because they consist of a metal bonded to a nonmetal. Properties of Carbonyl Compounds.
Ionic bonds form between two atoms that have different electronegativity valuesBecause the ability to attract electrons is so different between the atoms its like one atom donates its electron to the other atom in the chemical bond. Recognizing Compounds With Ionic Bonds. Inorganic compounds are those which are not organic that is they are without a carbon-hydrogen bond.
A structural formula is a visual representation that shows how atoms are arranged and bonded together in a chemical compound. CoorCoordinationdination Alfred Werner 1866-1919 a Swiss chemist was the first to formulate his ideas about the structures of coordination compounds. Covalent compounds metallic compounds and ionic compounds.
Organic Compounds Definition Organic compounds are a type chemical compounds where one or more than one carbon covalently bonded with each other and with other atom like nitrogen oxygen halogen etc. He prepared and characterised a large number of coordination compounds and. The atoms in a molecule of water have formed very simple bonds due to this lack of carbon.
Compounds can be of three types which are. Many inorganic compounds are ionic compounds consisting of cations and anions joined by ionic bondingExamples of salts which are ionic compounds are magnesium chloride MgCl 2 which consists of magnesium cations Mg 2 and chloride anions Cl. These are to be polar in nature.
Or sodium oxide Na 2 O which consists of sodium cations Na and oxide anions O 2In any salt the proportions of the ions are such that the. Mixtures are mainly of two types ie. Inorganic compounds are not derived from living things.
Meanwhile you can simply say that inorganic compounds are just the opposite. They exhibit both positive and negative charge in slight form. Most of the inorganic compounds do not contain carbon nor have C-H bonds.
HCl - Hydrochloride also known as hydrochloric acid when it is dissolved in water is a colorless corrosive acid with a fairly strong pH. Coordination Compounds are the backbone of modern inorganic and bioinorganic chemistry and chemical industry. Examples of Organic compounds uses come from living thing and are said to be compounds consisted C-H.

Organic Or Inorganic Texas Gateway
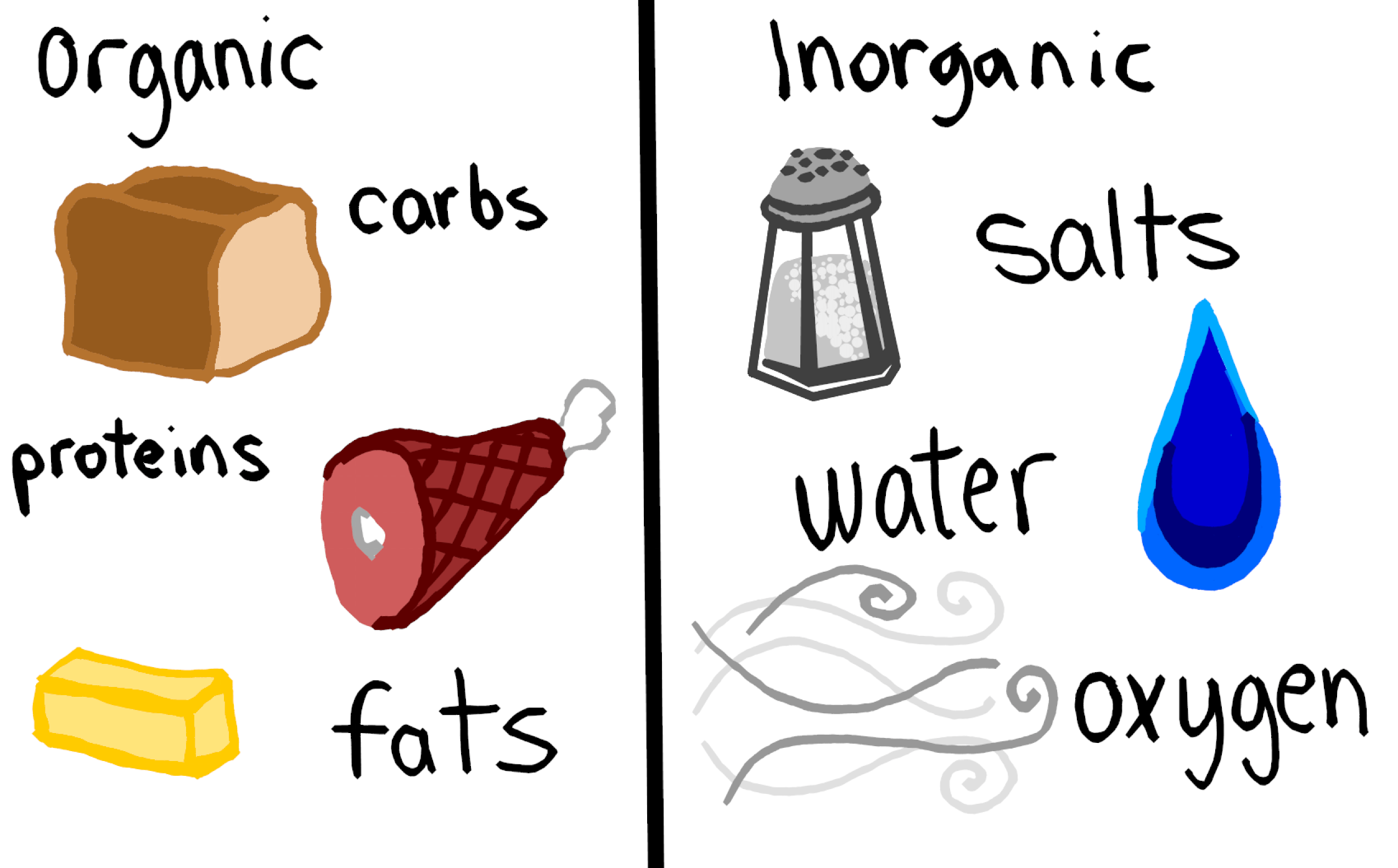
Organic Vs Inorganic Molecules Definition Overview Expii

Organic Or Inorganic Texas Gateway

List Of Inorganic Chemicals Used In The Experiments Download Table

Inorganic Compounds Essential To Human Functioning Anatomy And Physiology I

Inorganic Compounds An Overview Sciencedirect Topics

What Are Inorganic Compounds Definition Characteristics Examples Video Lesson Transcript Study Com
Organic Vs Inorganic Compounds Definition 13 Key Differences Examples

Organic Vs Inorganic Molecules Definition Overview Expii

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Study Chemistry

Examples Of Organic Compounds And Inorganic Compounds In Hindi Urdu Youtube Youtube
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Organic Or Inorganic Texas Gateway
14 Difference Between Organic And Inorganic Compounds With Examples Viva Differences

Inorganic Compound Chemical Compound Britannica
Difference Between Organic And Inorganic Compounds
Difference Between Organic And Inorganic Compounds Key Differences

Examples Of Metals And Inorganic Compounds Used In Medicine Rat Oral Download Scientific Diagram

Examples Of Metals And Inorganic Compounds Used In Medicine Rat Oral Download Scientific Diagram